Abstract
Oxidative stress in adipose tissue may alter the secretion pattern of adipocytokines and potentially promote atherosclerosis. However, the therapeutic role of hydrogen in adipose tissue under oxidative stress remains unclear. In this study, subcutaneous adipose tissue (SCAT) was collected from the mid-thoracic wounds of 12 patients who underwent open-heart surgery with a mid-thoracic incision. The adipose tissue was then immersed in a culture medium dissolved with hydrogen, which was generated using a hydrogen-generating device. The weight of the adipose tissue was measured before and after hydrogenation, and the tissue was immunostained for nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), and superoxide dismutase (SOD), which are markers of oxidative stress. The immunostaining results showed that HO-1 and Nrf2 expression levels were significantly decreased in the hydrogenated group, whereas SOD expression levels increased, but did not attain statistical significance. Image analysis of adipose tissue revealed that a reduction in adipocyte size. Furthermore, hydrogenated adipose tissue showed a trend toward increased gene expression levels of adiponectin and decreased gene expression levels of chemerin, an adipocytokine involved in adipogenesis. These results demonstrated the therapeutic potential of hydrogen gas for oxidative stress in adipose tissue and for reducing adipocyte size.
Similar content being viewed by others
Introduction
Molecular hydrogen (H2) is a colorless, odorless gas, and the lightest and most abundant chemical element. Hydrogen gas is a weak reducing agent, and its oxidation-reduction reaction occurs only in the presence of a potent oxidant that causes tissue damage1. Due to its low molecular weight, hydrogen can easily permeate through cell membranes and reach the mitochondria, where ROS are produced, or the nucleus, where genetic information is stored, thereby protecting these organelles from oxidative damage2,3. In recent years, studies have been conducted on the capacity of hydrogen to mitigate oxidative stress, highlighting its potential preventive and therapeutic benefits. It is considered an effective antioxidant that can reduce oxidative stress1. In cardiovascular surgery, the main function of hydrogen is to decrease ischemic action, potentially preventing atherosclerosis. It was also noted that the aging of vascular endothelial cells associated with atherosclerotic lesions was suppressed in mice that drank highly concentrated hydrogen water4. However, many questions persist regarding its precise mechanism, particularly concerning its role in adipose tissue.
Adipose tissue is one of the most significantly impacted by reactive oxygen species (ROS) imbalance. In adipose tissue, high nutrient ingestion leads to ROS generation5. Oxidative stress in adipose tissue has been implicated in altering the secretion profile of adipocytokines, potentially exacerbating conditions such as atherosclerosis, a prevalent cardiovascular disease characterized by inflammatory cell infiltration and lipid accumulation in major arteries due to excessive ROS6. Furthermore, vascular dysfunction could be prevented and treated by focusing on oxidative stress in adipose tissues. Increased ROS production in obesity is intimately linked to the dysregulation of adipocytokine expression in adipose tissues7, a major endocrine organ8, comprising various cellular components, including adipocytes, which synthesize and secrete a range of biologically active molecules termed adipocytokines. These molecules provide critical functions in regulating systemic metabolism and inflammation.
Adiponectin, another adipocytokine, is present at low levels in patients with obesity and diabetes mellitus9. Adiponectin regulates substrate metabolism and vessel wall health, with HDL-cholesterol and high-molecular-weight adiponectin levels being positively associated, while total serum and high-molecular-weight adiponectin levels are negatively correlated with triglyceride and inflammatory marker levels10,11.
Chemerin has also been identified as an adipocytokine secreted by adipose tissue12,13. It is a chemotactic factor for dendritic cells and macrophages, promotes adipogenic differentiation, and exhibits angiogenic potential both in vivo and in cultured endothelial cells14. Chemerin and chemokine-like receptor 1 (CMKLR1) are highly expressed in the adipose tissue, predominantly in mature adipocytes15. Plasma chemerin levels are increased in patients and animals with obesity, coronary artery disease, and type 2 diabetes and are correlated with insulin resistance15,16,17,18,19,20.
Several enzymes, such as SOD and HO-1, could decrease ROS levels and function as an antioxidant defense. The adipocyte-specific overexpression of HO-1 reduced high-fat diet-induced adiposity and vascular dysfunction while enhancing insulin sensitivity and adipocyte function by regulating adiponectin levels and inflammation21. SOD catalyzes the dismutation of superoxide radicals into less harmful species22, whereas HO-1 produces antioxidant and anti-inflammatory compounds by breaking down heme23. These cytoprotective processes are crucial for maintaining cellular homeostasis, particularly under cellular stress24,25,26.
Nrf2 is also a transcription factor that plays a key role in antioxidant system regulation27. Under normal conditions, Nrf2 binds to the Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm. In response to oxidative stress, Nrf2 translocates to the nucleus where it binds to the antioxidant response elements. Nrf2 enhances the expression of several cytoprotective genes, including the antioxidant enzymes SOD28 and HO-129. Nrf2 is highly expressed in the adipose tissue30, but its exact function in adipocyte biology remains unknown.
In this study, we investigated the effects of hydrogen on adipose tissue, focusing on its potential to modulate oxidative stress reactions, regulate adipocytokine expression, and affect the morphological features of adipose tissue ex vivo.
Results
Patient characteristics
This study collected subcutaneous adipose tissue from 12 patients who underwent open-heart surgery via a midline sternotomy approach. The characteristics of the 12 study participants are presented in Table 1. The patients comprised 7 (58%) males and 5 (42%) females, with a mean age of 71.3 ± 7.9 years, and an average body mass index (BMI) of 23.8 ± 1.8 kg/m2. The frequencies of hypertension, dyslipidemia, overweight, and diabetes mellitus were 33% (n = 4), 8% (n = 1), 42% (n = 5), and 42% (n = 5), respectively.
Measurement of hydrogen concentration
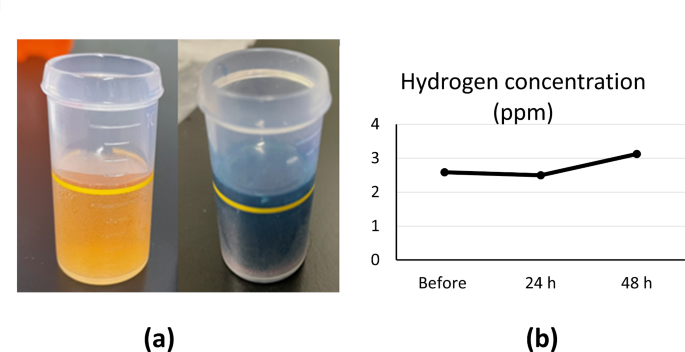
To ensure sufficient hydrogen was dissolved in the culture medium for soaking the adipose tissue, the hydrogen concentration was measured using a dissolved hydrogen concentration measurement reagent from Miz Corporation (Tokyo, Japan). The hydrogen concentration was measured by adding 6 mL of the reagent into the medium using the titration method (Fig. 1a). The number of drops required to change the medium from yellow to dark blue was used to calculate the dissolved-hydrogen concentration, which averaged 2–3 PPM. The hydrogen concentration was 2.58 PPM before hydrogenation. After 24 h, it was adjusted to 2.5 PPM with a hydrogen generator and remained high after 48 h, with an average of 3.13 PPM. After 48 h of hydrogenation, the concentration of hydrogen in the culture solution increased notably compared to the previous two measurements (Fig. 1b).
Measurement of adipose tissue weight
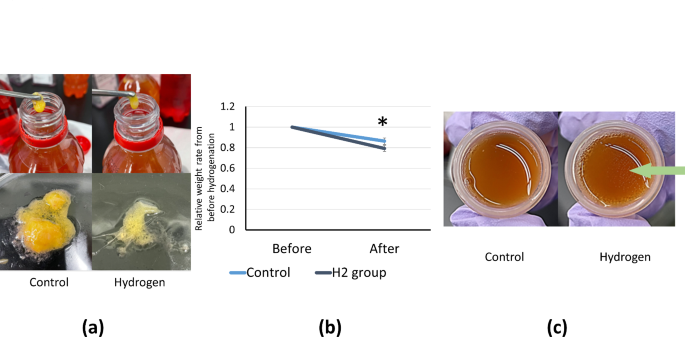
In all cases, tissues taken from patients were divided into two portions and placed in a hydrogenated and control medium, respectively, and morphological changes were examined after 48 h. After hydrogenation, more adipose tissue was dissolved in the culture solution than in the control culture solution (Fig. 2a). The weight of the adipose tissue was significantly lower in the hydrogenated group than in the control group. The rate of decrease in adipose tissue weight was also assessed. The control group displayed a slight reduction, whereas the hydrogen-exposed group showed a measurable increase in the rate of decrease. After hydrogenation, the average reduction in the adipose tissue weight increased from 13.7 to 20.1%. (Fig. 2b). Owing to the high water content of the culture medium, the dry weight may show an even more pronounced difference. Additionally, fat droplets floating on the entire surface of the culture medium were observed in the hydrogenated group (indicated by the arrow), whereas they were not observed in the control group (Fig. 2c).
Measurement of adipose tissue and adipocyte size
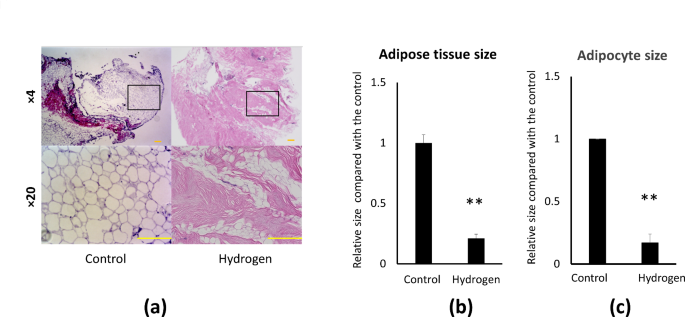
To assess if hydrogen causes morphological changes in adipose tissue and adipocytes at microscopic level, adipose tissue and adipocyte sizes were determined using ImageJ software (National Institutes of Health, Bethesda, MD, USA) (Fig. 3a). After hydrogenation, there was a 78.9% reduction in adipose tissue size (Fig. 3b) and an 83% decrease in adipocyte size in the hydrogenated group compared to the control group (Fig. 3c).
Effect of hydrogenation on Nrf2, HO-1, and SOD expression
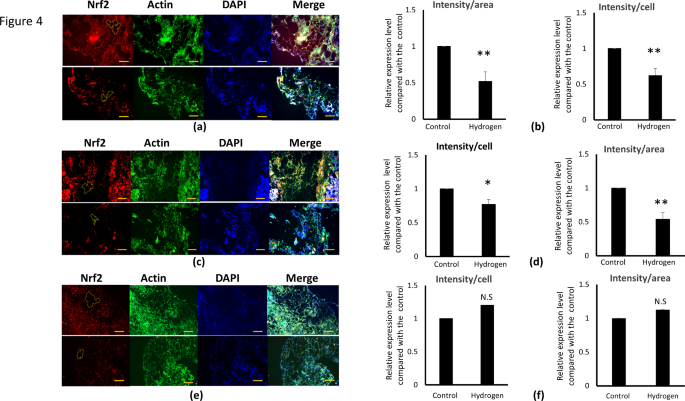
The effects of hydrogenation on oxidative stress were investigated by examining changes in representative oxidative stress markers, Nrf2, HO-1, and SOD, using immunofluorescence staining. Immunostaining revealed a notable decrease in the expression levels of Nrf2 and HO-1, accompanied by reduced adipocyte size in the hydrogenated group. Conversely, although SOD expression levels increased, no significant differences were observed. The fluorescence intensity of the adipose tissue per cell and area was quantified using ImageJ software (Fig. 4a, c, e). The differences in Nrf2 and HO-1 expression levels after 48 h of hydrogenation are depicted in Fig. 4a and c, indicating lower levels in the hydrogenated group than in the control group. Both the cell count and cell area ratio were markedly lower in the hydrogenated group (Fig. 4b, d). These graphs show the percentage changes in Nrf2 and HO-1 expression levels in 12 patients, with the relative expression in the hydrogenated group compared to the control set at 1. After hydrogenation, Nrf2 expression levels exhibited a 38% reduction in intensity per cell and a 48% decrease in intensity per area (Fig. 4b). HO-1 expression decreased by 23% and 46% in terms of intensity per cell and area, respectively (Fig. 4d). The graph delineates the percentage change in SOD expression in 12 patients, with the relative expression in the hydrogenated group displayed alongside the control set at 1. After hydrogenation, we observed an increase in the SOD expression levels of 20% in intensity per cell and by 12.6% in intensity per area. SOD levels vary from patient to patient, with some cases showing an increase and others showing a decrease. Overall, no significant trend was observed in the average percentage increase (Fig. 4f).
Immunohistochemical staining of adipose tissue to determine the expression levels of Nrf2, HO-1, and SOD in adipose tissue. (a, c, e) Red indicates Nrf2 (a), HO-1 (c), and SOD (e) stained with Alexa Flour 594, blue indicates nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI), and green indicates actin stained with Alexa Flour 488-phalloidin to stain the surrounding tissue. From the green area, the area containing only adipocytes was extracted, and the fluorescence intensity of the adipose tissue per cell number and area was quantified using Image J, with measurements taken along the yellow line within the red section. These graphs show the percentage change in Nrf2, HO-1, and SOD expression levels in 12 patients, with the relative expression in the hydrogenated group compared to the control set at 1. The result is shown as the mean ± SE. * p < 0.05, ** p < 0.01 versus control. Scale bar, 500 μm.
Evaluation of adipocytokine gene expression by quantitative PCR
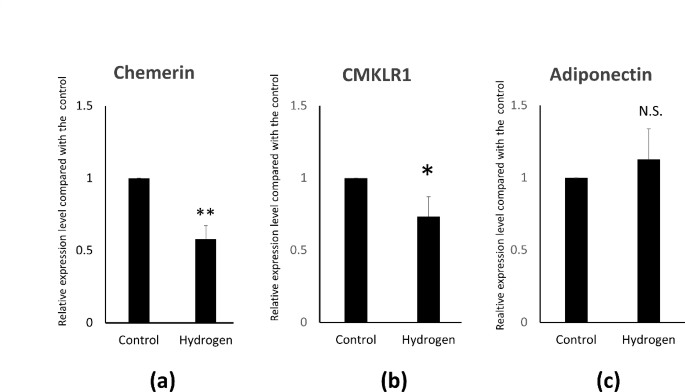
To investigate the effect of hydrogen on the adipocytokine gene in adipocytes, the expression of adipocytokines was measured using quantitative polymerase chain reaction (qPCR) three hours after hydrogenation. A 42% decrease in chemerin expression (Fig. 5a), and a corresponding 26.7% decrease in CMKLR2 expression levels were observed (Fig. 5b). Conversely, adiponectin expression increased by 13%, although the difference was not significant (Fig. 5c).
Discussion
This study represents the first use of ex vivo adipose tissue samples from cardiac surgery to explore changes in the expression of oxidative stress markers and adipocyte size. In this study, we aimed to elucidate the effects of hydrogenation on these parameters and the potential therapeutic role of hydrogenation in adipose tissue physiology. It should be mentioned that the adipose tissue specimens were obtained after surgery under general anesthesia, which is different from the usual in vivo conditions. We used the SCAT obtained from under the sternal area with the patient’s consent. During cardiac surgery, SCAT is easier to obtain than epicardial adipose tissue (EAT), pericardial adipose tissue (PCAT) and periaortic adipose tissues (PAAT). The experimental findings revealed a drastic reduction in the expression levels of Nrf2 and HO-1 and reduced adipocyte size following hydrogenation. During the preparation process for the hydrogen administration experiment, direct exposure of intraoperative adipose tissue samples revealed new findings. These findings provide potential insights into the effects of hydrogen on the adipose tissue and shed light on its therapeutic role in reducing fat accumulation and altering adipocyte metabolism.
Several studies have shown that the expression of Nrf2 and HO-1 alleviates oxidative stress. The finding that the addition of hydrogen gas caused a significant decrease in Nrf2 and HO-1 expression levels ex vivo suggests that hydrogen gas directly reduced ROS levels or decreased Nrf2 and HO-1 expression levels. Alternatively, hydrogen gas may have caused a broad relief from oxidative stress. However, the effect of hydrogen gas and the mechanism behind decreased expression of these oxidative stress markers could not be directly demonstrated in this experiment. The detailed mechanisms of hydrogen gas’s effect on oxidative stress and the clinical significance of hydrogen gas are topics for future research.
Some studies have shown that Nrf2 deficiency decreases the expression of target antioxidant genes31. Hydrogen can reduce the oxidative damage caused by ROS, even in the absence of Nrf232. Furthermore, Nrf2-knockout mice showed markedly decreased adipose tissue mass and adipocyte size33.
Hydrogen-rich saline monotherapy decreases HO-1 expression levels34. As stressful conditions significantly enhance HO-1 expression and activity, a primary role of HO-1 is implicated in antioxidant and anti-inflammatory responses35,36. In contrast, while the addition of hydrogen gas suppressed SOD expression in some cases, no significant decrease or trend was observed in others. It is difficult to determine the clinical criteria influencing the inhibitory effect of hydrogen gas on SOD expression. Some studies have shown that hydrogen treatment increases SOD activity of SOD37,38,39,40. Patients with coronary artery disease exhibit higher SOD expression during the early stages of the disease. Nevertheless, SOD expression was downregulated as the disease proceeds41. SOD may be expressed permanently under high-stress conditions independent of Nrf2 expression levels. In the hydrogenated group, SOD expression was maintained even after a decrease in Nrf2 and HO-1 expression levels was observed. Incidentally, there was a trend toward decreased mRNA expression of Nrf-2 and HO-1 by hydrogen exposure; no significant difference was observed (supplemental figure). This discrepancy in these results may be due to the action of post-translational long non-coding RNA or the inhibition of protein degradation in the ubiquitin-proteasome system by hydrogen; the latter experiment was inconclusive.
Although hydrogen addition may have a major effect on certain pathways, its involvement in all oxidative stress pathways remains unclear. Upon hydrogenation, we observed a considerable reduction in adipocyte size. However, the mechanism by which hydrogen gas reduces the adipocyte size remains unclear. Morphological alterations in the lipid bilayer in the cell membrane of adipocytes are thought to cause the fat inside the cell to disintegrate; however, the process is currently uncertain. Although some pressure is generated in the gas phase during hydrogen gas production by sealing with a plastic container, the effect of pressure is considered minor because the adipose tissue is present in the liquid phase. Hydrogen may physically reduce the size of adipocytes by altering the behavior of the lipid bilayer. It is possible that the reduction in adipocyte size was not solely attributable to the decreased expression level of Nrf2, and that additional factors were involved. Further studies are required to elucidate the precise mechanisms underlying this phenomenon and its clinical significance. Hydrogen gas can be obtained using two methods: directly purchasing hydrogen gas filled in cylinders, or dissolving hydrogen in a solvent using hydrogen-generating equipment, as demonstrated in this experiment. The hydrogen-generating device has the advantage of producing a liquid comprising dissolved hydrogen with a maximum observed value of 3 PPM. As the amount of hydrogen gas produced is modest, the risk of igniting an explosion is extremely low, making it safe for use. Although hydrogen gas has been used almost exclusively for therapeutic purposes, its clinical application has gained momentum in recent years owing to its antioxidant properties. In the field of cardiovascular surgery, clinical trials using hydrogen gas to protect against organ damage and increase survival rates are ongoing. Drinking water with a high concentration (1.2–1.6 PPM) has a neuroprotective effect in mice42. Hydrogen-rich water (hydrogen concentration: 1.9 PPM) is used as a supplement to help athletes improve their performance and reduce weariness43.
Furthermore, we did not investigate how inhibition of Nrf2 and HO-1 expression affects PCAT and PAAT, both of which are thought to be influenced by atherosclerosis. In the future, we will examine the adipocytokine expression levels in EAT, PCAT, and PAAT, which may be significantly altered by hydrogenation. The clinical impact of adipocyte shrinkage is beneficial in aesthetic medicine, and a living organism was used in this study to demonstrate that adipose tissue shrinkage occurs upon direct exposure to hydrogen gas. However, because of the high concentration of hydrogen gas and the long exposure time of 48 h, this experiment was limited to ex vivo investigations and thus differed from actual clinical applications. The fact that this oxidative stress decreases not only in adipocytes but also in adjacent fibroblasts, shows that hydrogen gas may have a wide variety of clinical applications beyond cardiac surgery.
Using qPCR, we analyzed the gene expression levels of several cytokines that may be involved in atherosclerosis and observed a decreasing trend for chemerin and an increasing trend for adiponectin. Nevertheless, as different patients showed different shifts, only chemerin showed significant differences. Interleukin 1 beta and tumor necrosis factor-alpha levels were not significantly different (data not shown). Adiponectin is exclusively secreted from the adipose tissue, and its expression level is higher in SCAT than in visceral adipose tissue. Increased adiponectin levels reduce the adipocyte size and increase the adipocyte number44. These results suggest that the hydrogenation of adipocytokines may cause changes in adipocytokine expression; however, it is unclear whether these changes are significant. Chemerin and CMKLR1 are implicated in adipogenesis rather than functioning solely as adipocytokines. Therefore, when fat shrinkage is induced by hydrogen, it leads to a diminished capacity for adipogenesis, rather than simply reducing the presence of harmful cytokines. It is also associated with inflammation and oxidative stress. Another study demonstrated that hydrogen-rich/saline treatment (HRS) markedly reduced the expression level of chemerin following limb ischemia/reperfusion. HRS can ameliorate lung injury and reduce chemerin levels, and its protective effect is strongly associated with increased chemerin levels45. Incidentally, technical problems prevented us from assess accurate protein levels of adipocytokines by Western blot or ELISA.
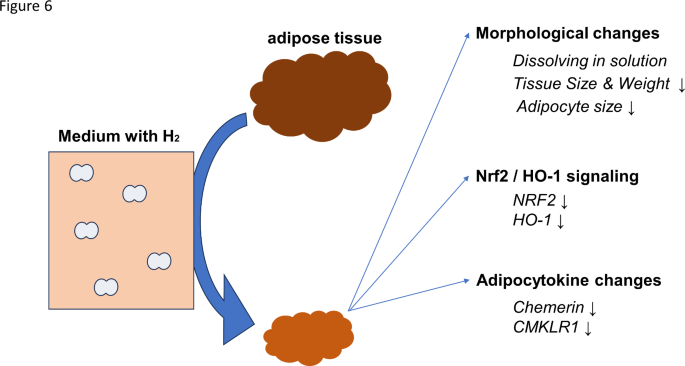
The components investigated in this study relate to phenomena relevant to the direct action of hydrogen on adipose tissue ex vivo, and it should provide significant information for future hydrogen research (Fig. 6).
The limitations of this study are:
‣We could not make conclusions about the correlation between various data related to hydrogenation and other factors, such as sex and age. Moreover, these findings were derived from a small number of cases.
‣We could not find an association between all the constructs studied based on the results of this survey. Proving these associations and mechanisms is a subject for future research. Further studies are required to address these issues.
In conclusion, our findings suggest that hydrogen gas mitigates oxidative stress responses in the adipose tissue and reduces adipocyte size. These results highlight the potential clinical applications of hydrogen gas in the context of adipose tissue biology and oxidative stress-related conditions.
Methods
Subjects
The study protocol was approved by the Ethics Committee of Nagoya City University Hospital. We recruited 12 subjects aged 21 years and older, including seven males and five females. Subcutaneous adipose tissue was collected from patients who underwent open-heart surgery via a midline sternotomy approach and the adipose tissue was immersed in a culture medium containing a hydrogen-generating device at 37 °C for 48 h. The subjects were required to undergo a complete blood count test before surgery. Written informed consent was obtained from all participants prior to their enrollment in the study. All methods were performed in accordance with the relevant guidelines and regulations by including a statement.
Clinical parameters
Obesity was defined as BMI > 25 kg/m2 according to the criteria of the Japan Society for the Study of Obesity46. Diabetes mellitus was defined in accordance with the guidelines of the Japan Diabetes Society47. Hypertension was defined as systolic blood pressure ≥ 140 mmHg and diastolic BP ≥ 90 mmHg48.
Hydrogen solution preparation and concentration measurement
The day before the experiment, 500 mL of Dulbecco’s modified Eagle’s medium (low glucose) supplemented with L-glutamine and phenol red (FUJIFILM Wako, Tokyo, Japan) was added to 25 mL of 5% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA) in a decanter. On the first day of hydrogenation, approximately 350 mL of the culture solution was poured into two polyethylene terephthalate (PET) bottles (soda bottles). The water tank was heated to 37 °C.
Preparation of PET bottles for the hydrogen group
An acrylic resin tube was filled with a nonwoven fabric (Miz Corporation, Ltd.) containing a hydrogen-generating agent. The tube was inserted into a PET bottle filled with the culture solution and tightly closed with a cap attached to a check valve. Hydrogen gas was released into water through a check valve attached to an acrylic resin tube. According to the manufacturer’s instructions, it can generate up to six atmospheres; however, actual measurements revealed that it was nearly equivalent to atmospheric pressure, owing to leakage from the PET bottle cap.
Determination of the dissolved hydrogen concentration
The hydrogen concentration was determined over time using the Miz Corporation’s dissolved hydrogen concentration measurement reagent. Three hydrogen concentration measurements were performed on the first day before introducing the adipose tissue, 24 h later, and 48 h later. Six milliliters of the hydrogenated solution were collected in a cup. Twenty drops of the hydrogenation concentration determination reagent were added to the cup, one drop at a time, and the solution was mixed. The solution contained more hydrogen than methylene blue (MB) early in the titration process (the MB colloidal platinum (Pt) reagent was poured into hydrogen-rich water). Consequently, MB was converted to leuco-MB, and the resulting solution was colorless. The solution contained more MB than hydrogen at the titration endpoint, resulting in a blue color. It contained hydrogen (approximately 3 PPM) generated by a hydrogen-generating device (Miz Corporation).
Immunohistochemistry analysis
After 48 h of hydrogenation, the adipose tissue was fixed in 3% formalin in phosphate-buffered saline (PBS) overnight at 4 °C. Sections were prepared for immunohistochemical analysis. The tissues were embedded in paraffin and sectioned. Paraffin-embedded sections were deparaffinized with xylene and ethanol, washed three times with PBS, blocked with 1% bovine serum albumin, and permeabilized with 0.05% Triton. The sections were then incubated overnight at 4 °C with anti-Nrf2 (Bioss, Woburn, MA, USA), anti-HO-1 (Abcam, Cambridge, UK), or anti-SOD (Abcam) primary antibodies diluted at 1:200. The next day, the sections were washed three times with PBS and incubated with secondary antibodies overnight at 4 °C for immunofluorescence analysis. They were then stained with DAPI (Sigma-Aldrich, St Louis, MO, USA), Alexa flour 488-phalloidin (Thermo Fisher Scientific), and Alexa Fluor 594 (Invitrogen, Eugene, OR, USA) at a dilution of 1:1000. One drop of anti-fade mounting medium was added and coverslips were placed on the tissue sections. Images were captured using a fluorescence microscope (Carl Zeiss, Oberkochen, Germany). The fluorescence intensity per cell count and area in the adipose tissue images were calculated using the ImageJ software.
Determination of adipose tissue and adipocyte size
Adipose tissue and adipocyte size were determined from the captured images using ImageJ software. The fluorescence intensities per cell number and area were compared between the hydrogen and control groups.
qPCR analysis
After 48 h of adipose tissue hydrogenation, the tissue samples were fixed in an RNA stabilizer in PBS overnight at 4 °C to prepare for qPCR analysis. RNA was extracted from the adipose tissue using an RNeasy Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. cDNA was synthesized using ReverTra Ace (Toyobo, Osaka, Japan). Commercially available TaqMan assays (FAM-labeled), obtained from Thermo Fisher Scientific: ADIPOQ(adiponectin: Hs00505917_m1), RARRES2(chemerin: Hs00161209_g1), CMKLR1 (Hs00161209_m1), Nrf-2 (Hs00975961_g1), and HO-1(Hs01110250_m1) were used. The human beta-actin qPCR probe assay (Hs99999903_m1, VIC-labeled, Thermo Fisher Scientific) was used as an internal control. qPCR was performed using a Light Cycler 480 Instrument II (Roche, Basel, Switzerland).
Statistical analysis
Statistical significance was determined using an unpaired two-tailed Student’s t-test to compare two mean values. The results are expressed as the means SEM. p values less than 0.05 were considered significant.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. (Email: nnaka@dpc.agu.ac.jp).
Abbreviations
- SCAT: Subcutaneous adipose tissue
- Nrf2: Nuclear factor erythroid 2-related factor 2
- HO-1: Heme oxygenase-1
- SOD : Superoxide dismutase
- ROS : Reactive oxidative stress
- PAAT: Periaortic adipose tissue
- EAT: Epicardial adipose tissue
- KEAP1: Kelch-like ECH-associated protein 1
- ADIPOQ: Adiponectin
- CMKLR1: Chemokine-like receptor 1
- RARRES2: Retinoic acid receptor responder protein 2
References
-
Ohsawa, I. et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med.13, 688–694 (2007).
-
Sano, M. et al. Promising novel therapy with hydrogen gas for emergency and critical care medicine. Acute Med. Surg.5, 113–118 (2018).
-
Ostojic, S. M. Targeting molecular hydrogen to mitochondria: Barriers and gateways. Pharmacol. Res.94, 51–53 (2015).
-
Iketani, M. et al. Administration of hydrogen-rich water prevents vascular aging of the aorta in LDL receptor-deficient mice. Sci. Rep.8, (2018).
-
Santhakumar, A. B., Bulmer, A. C. & Singh, I. A review of the mechanisms and effectiveness of dietary polyphenols in reducing oxidative stress and thrombotic risk. J. Hum. Nutr. Diet.27, 1–21 (2014).
-
Björkegren, J. L. M. & Lusis, A. J. Atherosclerosis: Recent Developments. Cell. 185, 1630–1645 (2022).
-
Sakurai, T. et al. Exercise training attenuates the dysregulated expression of adipokines and oxidative stress in white adipose tissue. Oxid. Med. Cell. Longev. 9410954 (2017). (2017).
-
Wozniak, S. E., Gee, L. L., Wachtel, M. S. & Frezza, E. E. Adipose tissue: The new endocrine organ? A review article. Dig. Dis. Sci.54, 1847–1856 (2009).
-
Berg, A. H. & Scherer, P. E. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res.96, 939–949 (2005).
-
Aso, Y. et al. Comparison of serum high–molecular weight (HMW) adiponectin with total adiponectin concentrations in type 2 diabetic patients with coronary artery disease using a novel enzyme-linked immunosorbent assay to detect HMW adiponectin. Diabetes55, 1954–1960 (2006).
-
Bobbert, T. et al. Changes of adiponectin oligomer composition by moderate weight reduction. Diabetes54, 2712–2719 (2005).
-
Goralski, K. B. et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem.282, 28175–28188 (2007).
-
Nagpal, S. et al. Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin. (1997).
-
Nakamura, N. et al. Chemerin promotes angiogenesis in vivo. Physiol. Rep.6, e13962 (2018).
-
Bozaoglu, K. et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology148, 4687–4694 (2007).
-
Arita, Y. et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun.257, 79–83 (1999).
-
Koenig, W., Khuseyinova, N., Baumert, J., Meisinger, C. & Löwel, H. Serum concentrations of adiponectin and risk of type 2 diabetes mellitus and coronary heart disease in apparently healthy middle-aged men. J. Am. Coll. Cardiol.48, 1369–1377 (2006).
-
Parlee, S. D., McNeil, J. O., Muruganandan, S., Sinal, C. J. & Goralski, K. B. Elastase and tryptase govern TNFα-mediated production of active chemerin by adipocytes. PLoS ONE7, e51072 (2012).
-
Yamauchi, T. et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med.7, 941–946 (2001).
-
Pajvani, U. B. et al. Structure-function studies of the adipocyte-secreted hormone acrp30/adiponectin. J. Biol. Chem.278, 9073–9085 (2003).
-
Cao, J. et al. Heme oxygenase gene targeting to adipocytes attenuates adiposity and vascular dysfunction in mice fed a high-fat diet. Hypertension60, 467–475 (2012).
-
Yasui, K. & Baba, A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm. Res.55, 359–363 (2006).
-
Abraham, N. G. & Drummond, G. CD163-mediated hemoglobin-heme uptake activates macrophage HO-1, providing an antiinflammatory function. Circ. Res.99, 911–914 (2006).
-
Choi, A. M. & Alam, J. Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung Injury. Am. J. Respir Cell. Mol. Biol.15, 9–19 (1996).
-
Kangralkar, V. A., Patil, S. D. & Bandivadekar, R. M. Oxidative stress and diabetes: A review. in (2005).
-
Landis, G. N. & Tower, J. Superoxide dismutase evolution and life span regulation. Mech. Ageing Dev.126, 365–379 (2005).
-
Moi, P., Chan, K., Asunis, I., Cao, A. & Kan, Y. W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the β-globin locus control region. Proc. Natl. Acad. Sci. U S A91, 9926–9930 (1994).
-
Cominacini, L. et al. Endoplasmic reticulum stress and Nrf2 signaling in cardiovascular diseases. Free Radic Biol. Med.88, 233–242 (2015).
-
Wang, L. J., Lee, T. S., Lee, F. Y., Pai, R. C. & Chau, L. Y. Expression of heme oxygenase-1 in atherosclerotic lesions. Am. J. Pathol.152, 711–720 (1998).
-
Chan, K., Lu, R., Chang, J. C. & Kan, Y. W. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc. Natl. Acad. Sci.93, 13943–13948 (1996).
-
Miller, C. J. et al. Disruption of Nrf2/ARE signaling impairs antioxidant mechanisms and promotes cell degradation pathways in aged skeletal muscle. Biochim. Biophys. Acta BBA - Mol. Basis Dis.1822, 1038–1050 (2012).
-
Kawamura, T. et al. Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo. Am. J. Physiol. -Lung Cell. Mol. Physiol.304, L646–L656 (2013).
-
Pi, J. et al. Deficiency in the nuclear factor e2-related factor-2 transcription factor results in impaired adipogenesis and protects against diet-induced obesity. J. Biol. Chem.285, 9292–9300 (2010).
-
Jiang, Y. et al. Therapeutic efficacy of hydrogen–rich saline alone and in combination with PI3K inhibitor in non–small cell lung cancer. Mol. Med. Rep.https://doi.org/10.3892/mmr.2018.9168 (2018).
-
Lien, G. S. et al. Epidermal growth factor stimulates nuclear factor-κb activation and heme oxygenase-1 expression via c-Src, NADPHoOxidase, PI3K, and akt in human colon cancer cells. PLoS ONE9, e104891 (2014).
-
Nitti, M. et al. HO-1 induction in cancer progression: A matter of cell adaptation. Antioxidants6, 29 (2017).
-
Wang, L. et al. Hydrogen gas treatment improves the neurological outcome after traumatic brain injury via increasing miR-21 expression. Shock50, 308–315 (2018).
-
Ji, X. et al. Beneficial effects of hydrogen gas in a rat model of traumatic brain injury via reducing oxidative stress. Brain Res.1354, 196–205 (2010).
-
Xie, K. et al. Hydrogen gas improves survival rate and organ damage in zymosan-induced generalized inflammation model. Shock34, 495–501 (2010).
-
Huang, Y. et al. Beneficial effects of hydrogen gas against spinal cord ischemia–reperfusion injury in rabbits. Brain Res.1378, 125–136 (2011).
-
Gupta, S., Sodhi, S. & Mahajan, V. Correlation of antioxidants with lipid peroxidation and lipid profile in patients suffering from coronary artery disease. Expert Opin. Ther. Targets13, 889–894 (2009).
-
Igarashi, T. et al. Drinking hydrogen water improves photoreceptor structure and function in retinal degeneration 6 mice. Sci. Rep.12, 13610 (2022).
-
Timón, R. et al. Effects of 7-day intake of hydrogen-rich water on physical performance of trained and untrained subjects. Biol. Sport38, 269–275 (2021).
-
Kim, D. H. et al. Heme oxygenase-mediated Increases in adiponectin decrease fat content and inflammatory cytokines tumor necrosis factor-α and interleukin-6 in zucker rats and reduce adipogenesis in human mesenchymal stem cells. J. Pharmacol. Exp. Ther.325, 833–840 (2008).
-
Zou, R. et al. Hydrogen-rich saline attenuates acute lung injury induced by limb ischemia/reperfusion via down-regulating chemerin and NLRP3 in rats. Shock52, 134–141 (2019).
-
The examination committee. Of Criteria for ‘obesity Disease’ in Japan, Japan Society for the Study of Obesity. New Criteria for `obesity Disease’ in Japan. Circ. J.66, 987–992 (2002).
-
The Committee of the Japan diabetes society on the diagnostic criteria of diabetes mellitus. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J. Diabetes Investig1, 212–228 (2010).
-
Umemura, S. et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens. Res.42, 1235–1481 (2019).
Funding
This research was funded in part by a Grant-in-Aid for Scientific Research (21592506) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT).
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the Human Research Ethics Committee of the Ethics Committee of Nagoya City University Hospital. (60-20-0059), and each participant provided written informed consent for participation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.